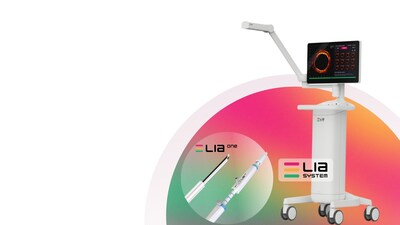
SAN JOSE, Calif., Feb. 5, 2026 /PRNewswire/ — LEADOPTIK, Inc., a medical technology company focused on advancing precision lung cancer biopsy, today announced the successful first-in-human clinical use of its FDA-cleared Last Inch Assessment™ (LIA) System at University of Chicago Medical Center.
This milestone follows the recent U.S. Food and Drug Administration (FDA) 510(k) clearance of LEADOPTIK’s LIA™ System [link to FDA clearance] and represents an important step toward validating its potential to improve diagnostic accuracy during lung biopsy procedures.
The first-in-human case was performed using the LIA™ system’s integrated optical imaging, which provides real-time, depth-resolved insights at the point of tissue sampling. The technology is designed to help physicians confirm that biopsy tissue is collected from the intended target, addressing a longstanding challenge in lung cancer diagnosis.
“Based on this initial first-in-human experience, the LIA™System integrated seamlessly into the clinical workflow and provided real-time intra-tool lesion confirmation during tissue sampling,” said D. Kyle Hogarth, M.D., Professor of Medicine and Director of Interventional Pulmonary and Advanced Bronchoscopy at the University of Chicago. “While early, the initial accuracy observed was highly encouraging and reinforced confidence at the point of biopsy.”
“Treating our first patients marks a defining moment for LEADOPTIK,” said Reza Khorasaninejad, Ph.D., CEO and Co-Founder of LEADOPTIK. “After years of development and encouraging preclinical data, we are now translating this technology into early clinical experience. Our goal is to help clinicians make more confident decisions and help patients get answers sooner. In the first six patients, LIA was able to differentiate nodules from healthy tissue in real time, something that has not been possible before.”
Dr. Hogarth, a pioneer in the field and a clinical leader in robotic bronchoscopy, added, “When I first heard the vision from the founders, I immediately recognized the potential to address the missing link between navigation and pathology in lung biopsy during the biopsy. This first-in-human case reinforced that belief and increased my excitement to help advance this technology to the next level.”
“Even in these early cases, we are learning how real-time tissue confirmation fits naturally into existing biopsy workflows,” said Alex Chee, M.D., Chief Medical Officer of LEADOPTIK. “These insights will directly inform future clinical studies and the development of our advanced software and AI-based tissue characterization capabilities.”
“This first patient treatment represents a critical step forward,” said Nick Byron, Head of Business Development at LEADOPTIK. “We look forward to expanding our limited launch and continuing to work with physicians at leading medical centers and hospitals to accelerate access to this technology, helping more patients receive timely and definitive answers from their first biopsy.”
Lung cancer remains the leading cause of cancer-related death worldwide. While navigational and robotic bronchoscopy technologies have improved access to pulmonary nodules, confirming successful tissue sampling remains a critical unmet need.
LEADOPTIK’s LIA™ System is designed to address this final step, the “last inch”, where procedural precision can directly impact diagnostic confidence and downstream patient care.
About LEADOPTIK:
LEADOPTIK is a Silicon Valley–based company transforming biopsy into a digital tissue-intelligence workflow. By integrating proprietary depth-resolved imaging technology with emerging analytics, LEADOPTIK’s Last Inch Assessment™ (LIA) system bridges the gap between navigation and pathology, enabling more precise and informed decision-making in interventional pulmonology and beyond.
For more information, visit www.leadoptik.com.
Media Contact:
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/leadoptik-announces-first-in-human-use-of-fda-cleared-lia-system-302680644.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/leadoptik-announces-first-in-human-use-of-fda-cleared-lia-system-302680644.html
SOURCE LEADOPTIK